HomeInnovation and DevelopmentGlobal Contract Manufacturing
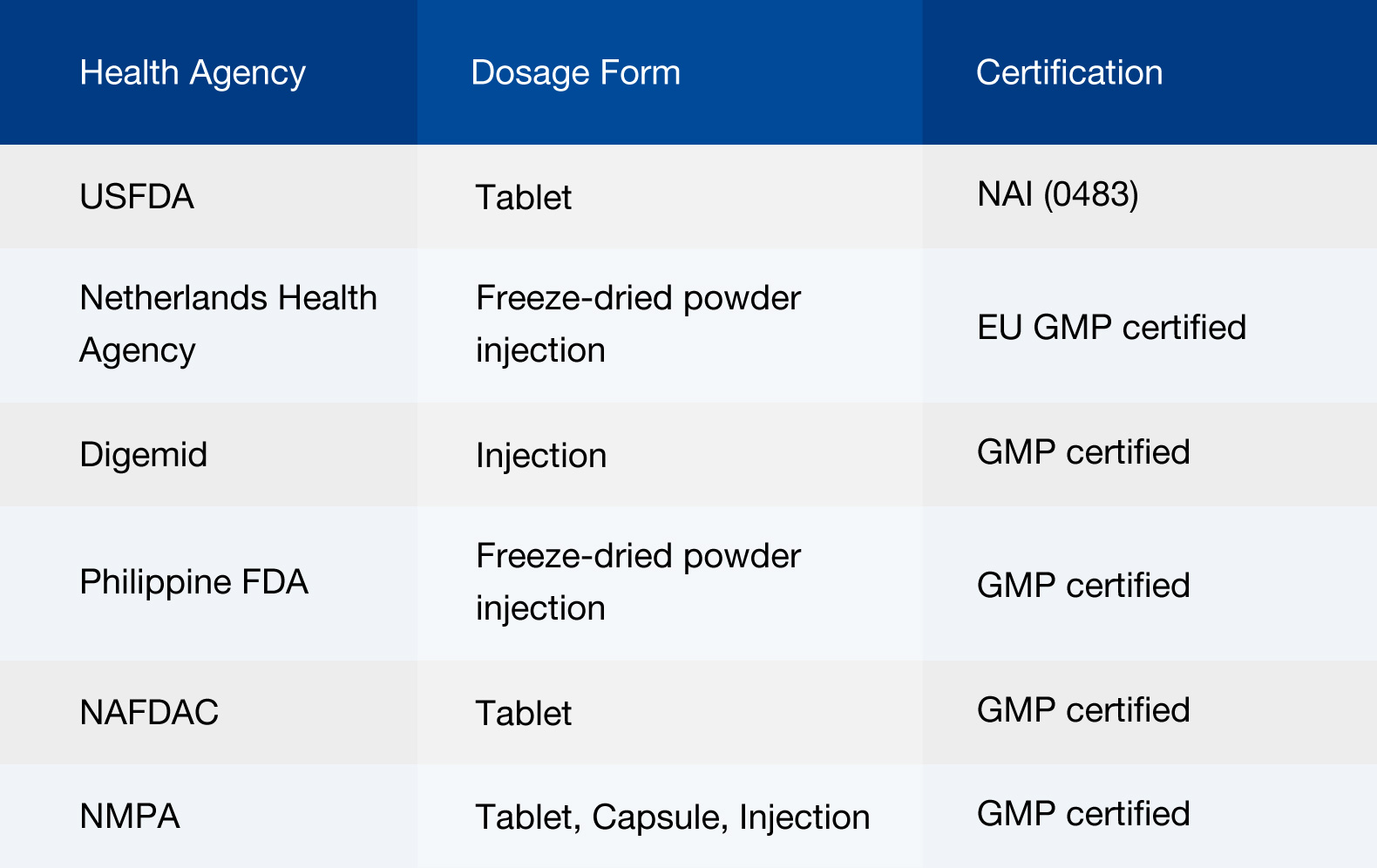
Products manufactured by Fosun Wanbang Pharma Group are exported to the countries of the United States, Germany, the Netherlands, Spain, Italy, Peru, Venezuela, Thailand, Malaysia and regions of Hong Kong and Taiwan.

GMP Site Inspection
At present, Fosun Wanbang Pharma Group has more than 10 production lines that have passed international site inspection, of which the freeze-dried powder injection production line passed the EU GMP inspection, and the OSD production line passed the US FDA GMP inspection.
Dosage Forms
Tablets
Capsules
Cloud Health
[separate production lines for ampoules, vials, cartridges and lyophilization]
Fosun Wanbang Pharma Group provide competitive advantages through advanced technology, quality control, lean production and global supply chain network. Our management is constantly reviewed and optimized to increase the use of the capacity, enhance supply chain performance, maintain high-level of customer service and decrease the waste for a better environment.
Tablets and Capsules can be packed in various blister and bottle packs according to customer requirement and the international packing serialization systems are installed to the production line.
Fosun Wanbang Pharma Group is honored to be a reliable contract manufacturing partner of the reputed multi-national companies for manufaturing of products for USA, Europe and other countries globally.
Tablets and Capsules can be packed in various blister and bottle packs according to customer requirement and the international packing serialization systems are installed to the production line.
Fosun Wanbang Pharma Group is honored to be a reliable contract manufacturing partner of the reputed multi-national companies for manufaturing of products for USA, Europe and other countries globally.